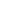In a landscape where bringing a new drug to market can require 10–15 years and incur costs exceeding $2.8 billion, the pharmaceutical industry is seeking more intelligent and faster solutions. Here’s where deep learning comes in, the transformative technology that’s revolutionizing how we conduct drug discovery, not by creating drugs from scratch, but by enabling us to repurpose existing drugs for new diseases.
A subset of artificial intelligence, deep learning, is currently fast-tracking drug repurposing by examining huge biomedical databases, revealing subtle trends and chronotypes, and making new predictions of drug-disease links at a pace and level of accuracy that have never been seen before. AI platforms have already been deployed to identify potential drugs for diseases, including COVID-19, Alzheimer’s, and cancer, with a shortened discovery time frame from years down to just weeks or months.
With 2,000+ approved drugs globally, the possibility of repurposing a fraction of them could provide cost-effective treatments, especially for rare and neglected diseases, and this possibility is now becoming a reality with deep learning.
In this blog, we’ll explore:
- How deep learning models are transforming drug repurposing
- Real-world success stories
- Benefits over traditional methods
- Challenges and ethical considerations
- The future of AI-driven drug discovery
What Is Drug Repurposing?
Drug repurposing, also known as drug repositioning, is the process of identifying new therapeutic uses for existing drugs. Instead of creating a new drug from whole cloth, researchers take a drug already approved for one disease and see if it might work for another. This approach has major benefits. Repurposing employs compounds with well-known safety profiles and methods of manufacture, dramatically reducing development times and risks.
For example:
The diabetes medicine metformin and the cholesterol medicine simvastatin were identified as promising for Alzheimer’s disease, as per recent studies. Repurposed drugs have well-established chemistry, with known dosing regimes, and can be moved quickly into new trials, occasionally saving years of work. In reality, between 20-30% of FDA-approved drugs receive at least one new indication after their initial approval. Put simply, drug repurposing is using what we already know to speed up getting treatments to patients.
Limitations Of the Traditional Drug Discovery Process
The traditional drug discovery pipeline is becoming unsustainable, both scientifically and economically. And it’s based on trial-and-error experiments, long timelines, and soaring costs. With the rapid evolution of the global burden of disease, particularly with rare and emergent diseases, the traditional approach is hard-pressed to keep pace.
Here’s why:
- Lengthy Timelines and Soaring Costs
It normally takes 10-15 years to develop a new drug and costs around US$1.3 2.6 billion, depending on how complicated and strict the regulations are. This prolonged process also delays needed care and depletes health resources. (life.bit)
- Staggering Failure Rates
Approximately 90% of candidate drugs fail in clinical trials due to a lack of efficacy or because of safety issues. But for all the preclinical research, most drugs never make it to patients, a massive bottleneck in innovation. (lifebit.ai)
- Low Incentive for Rare Diseases
There are more than 7,000 rare diseases, and only 5–7% of them have an FDA-approved treatment. Traditional models focus on blockbuster drugs, so niche or rare diseases are not well-served. (Nature)
- Unpredictable Safety & Efficacy Issues
Even when a compound is promising, it can fail because of unexpected toxicity or a lack of therapeutic effect in real-world settings, which lab models and animal trials frequently fail to predict.
Role Of Deep Learning In Drug Repurposing
Historically, drug repurposing was either based on serendipity or a single experimental observation. But now, deep learning — a cutting-edge type of Artificial Intelligence (AI) — is breaking that tradition wide open. By emulating the way the human mind makes sense of data, deep neural networks can sift through huge compendia of biological, chemical, and clinical information to reveal previously hidden connections. This technological leap is allowing researchers to predict what new uses existing drugs might have faster, cheaper, and more accurately than ever before.
1. Deep Learning Advantage in Large-Scale Pattern Recognition
Deep learning models are very good at recognizing complex, non-linear patterns from high-dimensional datasets. In the context of drug repurposing, this amounts to identifying relationships among:
- Drug molecular structures
- Genomic signatures of diseases
- Biological pathways and targets
- Real-world clinical outcomes
- Published biomedical literature
For example, Graph Neural Networks (GNNs) consider molecules as structured graphs of atoms and bonds. The models of these servers do learn how the composition of a drug features in its binding with biological targets and are thus very successful in virtual screening and the mechanism action on drug action of drugs.
2. NLP Architectures Research
The amount of biomedical literature doubles every 3-4 years, making it an impossible task for any human being to keep up with. But models like a natural language processing one can digest and comprehend millions of scientific papers, clinical trial data, and EHRs. Such AI tools enable the discovery of latent knowledge — off-label drug effects, contraindications, patient outcomes — that might take human researchers decades to discover.
Real-World Use-Case:
IBM Watson for Drug Discovery utilized NLP and deep learning to sift through over 100,000 scientific articles and identify new indications for six existing cancer drugs in a matter of weeks, compared to the months or years it would have taken researchers.
3. Analysis of Multiple Biomedical Data Types for Comprehensive Understanding
One of the most transformative capabilities of deep learning in biomedical research lies in its ability to integrate and analyze heterogeneous data types—including genomic sequences, molecular structures, clinical trial data, and electronic health records (EHRs).
By leveraging advanced architectures such as convolutional neural networks (CNNs), recurrent neural networks (RNNs), and transformers, deep learning models can detect intricate patterns, correlations, and anomalies across these datasets. These models:
- Decode protein sequences and molecular fingerprints to understand drug-target interactions
- Analyze patient health records to identify comorbidities and predict treatment responses
- Extract insights from clinical trial data to assess drug efficacy across populations
- Fuse omics data (genomics, proteomics, metabolomics) for precision medicine insights
This multi-modal analysis allows researchers to:
- Predict which existing or repurposed drugs may be effective against rare diseases
- Model complex interactions in polymicrobial or multifactorial diseases like cancer and Alzheimer’s
- Rapidly identify potential treatments during emerging pandemics, accelerating drug discovery timelines
4. Drug Discovery at Warp Speed, Speedier Thinking, and Leveraging Your Time
Thanks to deep learning, drug discovery has entered a whole new era, one where speed, precision, and personalization are no longer limited by traditional constraints.
Here’s how AI is reshaping the process:
- In silico screening now allows researchers to virtually analyze billions of chemical compounds in a matter of hours or days — something that would take years in a physical lab. This reduces both cost and time at the earliest, most critical stage of drug development.
- AI models can simulate how a specific patient’s body might respond to a particular drug based on their genetic and physiological data, leading to truly personalized medicine and reducing the risk of adverse reactions or inefficacy.
- Instead of starting from scratch, researchers are using AI to repurpose existing drugs, identifying new uses for old compounds without the need for time-consuming and expensive wet-lab trials right away. This fast-tracks treatments, especially in urgent scenarios like pandemics.
The result?
- Faster R&D timelines
- Lower development costs
- Higher chances of finding effective, targeted treatments
In short, AI is giving the pharmaceutical world speedier thinking, sharper targeting, and smarter use of time and resources.
Benefits of Deep Learning In Drug Repurposing
As healthcare innovation races against time and rising costs, deep learning is reshaping drug repurposing with unprecedented speed and accuracy. By analyzing massive datasets and uncovering subtle drug-disease relationships, AI is helping scientists identify new uses for existing drugs faster, cheaper, and more reliably.
Here are the key benefits of using deep learning in drug repurposing:
- Speed & Cost Savings: Reusing already-approved drugs eliminates years from development. Deep learning speeds this process by automating the generation of hypotheses. Indeed, research indicates that repurposed drugs offer “accelerated timelines and lower costs” as well as “well-established safety profiles”. AI offers further efficiency gains: it can screen huge chemical libraries in minutes rather than years and predict toxicities without spending huge sums on trials.
- Increased Discovering Ability: AI, like machine learning, combines disparate forms of data all at once (things like chemical structures, genomics, patient histories, and literature). Such multimodal analysis enables the discovery of subtle drug–disease associations not detected using conventional analyses: AI could, for instance, mine clinical health records and research papers to discover that a drug unveils an unexpected benefit for a subgroup of a patient population.
- Increased Success Rate: The deep learning models are capable to learn the structure–activity relationships at highly complex levels. One report highlights how an AI-generated repurposing model discovered life-saving drugs for formerly untreatable diseases more quickly and accurately than did before (one was the then-experimental drug ruxolitinib).
- Leverage Proven Safety: Given that repurposed drugs have already undergone a safety assessment, many AI-identified candidates rapidly transition to new clinical testing. Approximately 30% of approved drugs have been found to have expanded uses over time by serendipity or analysis. AI is taking this wish and putting it into a system.
- Personalization and Precision: Deep learning enables tailoring therapies to patient subtypes. AI can use genomic and health data to recommend the best repurposed drug for a particular group of patients, advancing precision medicine.
For example, drug candidates effective on specific genetic or demographic subgroups can also be pinpointed by subgroup-aware AI frameworks, which are practically infeasible at scale without AI.
Challenges Of Deep Learning In Drug Repurposing
While deep learning has unlocked powerful opportunities in accelerating drug repurposing, the path to real-world application is far from smooth. Despite its ability to process vast datasets and uncover novel therapeutic insights, several limitations continue to slow down its clinical and commercial impact.
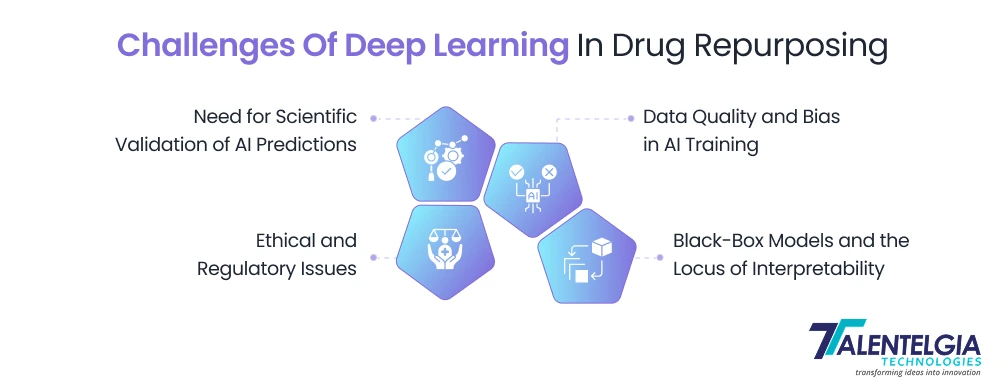
Here are the most critical challenges:
- Data Quality and Bias: AI models are only as good as the data they’re trained on. There is sufficient evidence that biomedical data are typically noisy, incomplete, or biased as well. There are, for instance, less represented populations in the clinical report, and rare side effects cannot be mentioned in the scientific databases. Poor data deep learning can find itself yielding misleading predictions. Students and scholars of these approaches acknowledge the need for usable/fit-for-purpose training data as well as standard training sets for enhancing AI reliability.
- Black-Box Models and the Locus of Interpretability: Many deep learning models (basically, anything you’d see in this post will be a form of neural network) are“black boxes”: it’s difficult to understand why the model made a particular prediction. For the field of medicine, where doctors and regulators demand lucid reasoning, this mystery is problematic. Explainable AI tools (such as SHAP or LIME) are beginning to help us rise to the challenge of showing us which of these features are influencing a prediction, but these are just additional complexity and still insufficient. Model transparency is vital to building trust and clinical integration.
- Ethical and Regulatory Issues: The use of patient data for decision-making carries the risk of privacy violation, and AI may also inadvertently learn biases (e.g., differences between sexes or ethnicities) that result in skewed recommendations. Regulatory bodies (FDA, EMA) are only now starting to define policies around AI in drug development. New FDA guidelines focus on solid clinical validation before any AI-led recommendations can be trusted. Any AI repurposing candidate still has to be tested in the real world.
- Scientific Validation: Eventually, we need to test AI predictions experimentally. An AI tool could identify a drug as a candidate, but biological systems are complex. Wetlab proof (cell assays, animal models) and controlled clinical trials are still necessary. So A.I. can speed up hypothesis generation, but that doesn’t get us out of the need for rigorous validation by science.
Also Read: Cost Of Implementing AI In Healthcare
Conclusion
The intersection of AI and precision medicine is the future of drug repurposing. Deep learning algorithms are also being developed that combine multi-omics data (genomics, proteomics, and metabolomics) with patient health records to enable truly personalized treatment regimens. For example, the tumor genome of a cancer patient could be analyzed by an AI, and an existing drug that targets that particular mutation could be repurposed. Powerful “foundation models” trained on vast knowledge in biomedicine (like TxGNN) can make zero-shot predictions, including for the rarest diseases. Health sensors that people will wear and the real-world data they supply will add a new feedback loop and enable AI to continuously refine the repurposing hypotheses in function of patient outcomes.
In this vision, AI not only speeds up repurposing but also makes it smarter by identifying “the one drug for the one patient.” With gene editing and biologics, AI-enabled repurposing could bring a new era of precision therapeutics that was previously unfathomable.


 Healthcare App Development Services
Healthcare App Development Services
 Real Estate Web Development Services
Real Estate Web Development Services
 E-Commerce App Development Services
E-Commerce App Development Services E-Commerce Web Development Services
E-Commerce Web Development Services Blockchain E-commerce Development Company
Blockchain E-commerce Development Company
 Fintech App Development Services
Fintech App Development Services Fintech Web Development
Fintech Web Development Blockchain Fintech Development Company
Blockchain Fintech Development Company
 E-Learning App Development Services
E-Learning App Development Services
 Restaurant App Development Company
Restaurant App Development Company
 Mobile Game Development Company
Mobile Game Development Company
 Travel App Development Company
Travel App Development Company
 Automotive Web Design
Automotive Web Design
 AI Traffic Management System
AI Traffic Management System
 AI Inventory Management Software
AI Inventory Management Software
 AI Software Development
AI Software Development  AI Development Company
AI Development Company  AI App Development Services
AI App Development Services  ChatGPT integration services
ChatGPT integration services  AI Integration Services
AI Integration Services  Generative AI Development Services
Generative AI Development Services  Natural Language Processing Company
Natural Language Processing Company Machine Learning Development
Machine Learning Development  Machine learning consulting services
Machine learning consulting services  Blockchain Development
Blockchain Development  Blockchain Software Development
Blockchain Software Development  Smart Contract Development Company
Smart Contract Development Company  NFT Marketplace Development Services
NFT Marketplace Development Services  Asset Tokenization Company
Asset Tokenization Company DeFi Wallet Development Company
DeFi Wallet Development Company Mobile App Development
Mobile App Development  IOS App Development
IOS App Development  Android App Development
Android App Development  Cross-Platform App Development
Cross-Platform App Development  Augmented Reality (AR) App Development
Augmented Reality (AR) App Development  Virtual Reality (VR) App Development
Virtual Reality (VR) App Development  Web App Development
Web App Development  SaaS App Development
SaaS App Development Flutter
Flutter  React Native
React Native  Swift (IOS)
Swift (IOS)  Kotlin (Android)
Kotlin (Android)  Mean Stack Development
Mean Stack Development  AngularJS Development
AngularJS Development  MongoDB Development
MongoDB Development  Nodejs Development
Nodejs Development  Database Development
Database Development Ruby on Rails Development
Ruby on Rails Development Expressjs Development
Expressjs Development  Full Stack Development
Full Stack Development  Web Development Services
Web Development Services  Laravel Development
Laravel Development  LAMP Development
LAMP Development  Custom PHP Development
Custom PHP Development  .Net Development
.Net Development  User Experience Design Services
User Experience Design Services  User Interface Design Services
User Interface Design Services  Automated Testing
Automated Testing  Manual Testing
Manual Testing  Digital Marketing Services
Digital Marketing Services 
 Ride-Sharing And Taxi Services
Ride-Sharing And Taxi Services Food Delivery Services
Food Delivery Services Grocery Delivery Services
Grocery Delivery Services Transportation And Logistics
Transportation And Logistics Car Wash App
Car Wash App Home Services App
Home Services App ERP Development Services
ERP Development Services CMS Development Services
CMS Development Services LMS Development
LMS Development CRM Development
CRM Development DevOps Development Services
DevOps Development Services AI Business Solutions
AI Business Solutions AI Cloud Solutions
AI Cloud Solutions AI Chatbot Development
AI Chatbot Development API Development
API Development Blockchain Product Development
Blockchain Product Development Cryptocurrency Wallet Development
Cryptocurrency Wallet Development About Talentelgia
About Talentelgia  Our Team
Our Team  Our Culture
Our Culture 
 Healthcare App Development Services
Healthcare App Development Services Real Estate Web Development Services
Real Estate Web Development Services E-Commerce App Development Services
E-Commerce App Development Services E-Commerce Web Development Services
E-Commerce Web Development Services Blockchain E-commerce
Development Company
Blockchain E-commerce
Development Company Fintech App Development Services
Fintech App Development Services Finance Web Development
Finance Web Development Blockchain Fintech
Development Company
Blockchain Fintech
Development Company E-Learning App Development Services
E-Learning App Development Services Restaurant App Development Company
Restaurant App Development Company Mobile Game Development Company
Mobile Game Development Company Travel App Development Company
Travel App Development Company Automotive Web Design
Automotive Web Design AI Traffic Management System
AI Traffic Management System AI Inventory Management Software
AI Inventory Management Software AI Software Development
AI Software Development AI Development Company
AI Development Company ChatGPT integration services
ChatGPT integration services AI Integration Services
AI Integration Services Machine Learning Development
Machine Learning Development Machine learning consulting services
Machine learning consulting services Blockchain Development
Blockchain Development Blockchain Software Development
Blockchain Software Development Smart contract development company
Smart contract development company NFT marketplace development services
NFT marketplace development services IOS App Development
IOS App Development Android App Development
Android App Development Cross-Platform App Development
Cross-Platform App Development Augmented Reality (AR) App
Development
Augmented Reality (AR) App
Development Virtual Reality (VR) App Development
Virtual Reality (VR) App Development Web App Development
Web App Development Flutter
Flutter React
Native
React
Native Swift
(IOS)
Swift
(IOS) Kotlin (Android)
Kotlin (Android) MEAN Stack Development
MEAN Stack Development AngularJS Development
AngularJS Development MongoDB Development
MongoDB Development Nodejs Development
Nodejs Development Database development services
Database development services Ruby on Rails Development services
Ruby on Rails Development services Expressjs Development
Expressjs Development Full Stack Development
Full Stack Development Web Development Services
Web Development Services Laravel Development
Laravel Development LAMP
Development
LAMP
Development Custom PHP Development
Custom PHP Development User Experience Design Services
User Experience Design Services User Interface Design Services
User Interface Design Services Automated Testing
Automated Testing Manual
Testing
Manual
Testing About Talentelgia
About Talentelgia Our Team
Our Team Our Culture
Our Culture

















 Write us on:
Write us on:  Business queries:
Business queries:  HR:
HR: