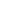In the complex realm of medical diagnostics, rare genetic diseases can be overlooked, misdiagnosed, misinterpreted, or missed altogether. These disorders, often the result of mutations in a single gene, are not as rare as many people think. GeneHome believes that there are around 10,000 different single-gene (monogenic) conditions. The World Health Organization puts the number of affected people as high as 10 in every 1,000. That is more than 70 million people around the world with a rare genetic disorder, and many have no diagnosis or plan for treatment. This blog explores how machine learning in Rare Genetic disorders is driving breakthroughs in early detection and diagnosis.
But what if the invisible could help to decode itself? What if there were algorithms that could sort through huge sets of genomic data? Also, find patterns that even the most well-trained clinicians might not notice?
This is in part where machine learning (ML) is coming into play. By helping to find unusual mutations and anticipate disease risks long before symptoms start. ML is fast emerging as a valuable weapon in the battle against diagnostic delays and unease.
Here’s the inside story of how machine learning is transforming the future of rare genetic disorder prediction, providing precision, speed, and life-saving hope to patients and practitioners.
What Are Rare Genetic Disorders?
Rare genetic disorders are health conditions caused by unusual changes or mutations in a person’s DNA and occur in a very small percentage of the population. Each disorder may affect only a few individuals worldwide, but collectively, rare genetic conditions impact millions of people. These disorders can influence how the body grows, functions, and repairs itself, sometimes with life-altering consequences.
Genetic conditions are the result of mutations or changes to a particular gene’s DNA. Such mutations can disrupt normal gene function or result in misjudging the quantity of genetic material in the body. Because genes are fundamental to the instructions for how cells grow, function, and repair themselves, even the tiniest change can be enough to set off serious health problems.
Half of your DNA comes from each parent. Some mutations are inherited across generations, while others can arise spontaneously through errors made during the copying process of DNA or under the influence of environmental factors. Symptoms may be present at birth or later in life, depending on the type and severity of the mutation.
With the advancement of technology, particularly in artificial intelligence and machine learning, researchers have a better ability to study these mutations. This could help predict rare genetic conditions before symptoms even appear, giving hope for improved diagnosis and treatment.
Why Conventional Methods Of Diagnosis For Rare Genetic Disorders Don’t Work?
Conventional methods of diagnosis for rare genetic disorders don’t work because they are time-consuming and costly, and often fail to yield diagnoses. This happens because of a step-by-step process, poor physician knowledge, and the fragmentation of health data. Taking this all in, patients often endure a “diagnostic odyssey,” slogging through family history reviews, physical exams, and a succession of increasingly fancy tests that can include gene panels, chromosomal microarrays, or genome sequencing—all with time-consuming expert interpretations required. Despite the progress in NGS, challenges remain, such as data analysis, variant interpretation, and testing availability, particularly in low- and middle-income countries.
Furthermore, ambiguous symptoms and disparate data retrieval also contribute to the time delay of accurate diagnostics, associated with increased costs and reduced patient well-being.
Key Limitations of Conventional Methods
Let’s go through the key limitations of conventional methods to diagnose genetic disorders,
1. Overlap of Symptoms with common diseases
Numerous rare genetic disorders present as the manifestation of common medical cases, resulting in misdiagnosis after misdiagnosis. Due to this lack of exposure or training among GPs, the signs and symptoms of rare diseases are usually dismissed or interpreted as something else.
2. Low Healthcare Provider Awareness
Health professionals usually have minimal or no training in rare genetic conditions, particularly in general practice. Patients thus bounce from one specialist to another, receiving no diagnosis, for years — a process known as the “diagnostic odyssey.”
3. Cost and Limited Availability of Specialized Testing
Genetic testing and the specialist assessments required to make a proper diagnosis are usually not available because of financial resources. Insurance barriers, expensive testing, and geographic challenges further limit diagnostic access, especially in resource-poor settings.
4. Complex Disease Presentation
Rare diseases frequently involve multiple organ systems and have manifestations at various times of life. This variation can make it difficult to recognize patterns by traditional means, particularly when detailed patient histories or genomic information isn’t immediately on hand.
5. The Isolated Data and Broken Records
The fragmented or incomplete patient data from hospitals impede comprehensive evaluations. These approaches are largely based on centralizing records, intuitive human analysis and interpretation, and do not work if the data is split or broken.
6. Time-Consuming Goals
Even with advanced testing, such as genome or exome sequencing, interpreting the results can take months. The absence of modern variant databases results in many positive findings being categorized as “variants of uncertain significance,” which do not have actionable results.
Firstly, let’s understand “What is Machine Learning?” so we can see how it connects to our main topic of discussion.
What Is Machine Learning?
Machine Learning (ML), a subfield of Artificial Intelligence (AI) in which algorithms are trained from data and sense patterns. AI makes decisions, or predictions, without being programmed explicitly. Its real power comes from learning over time as we accumulate experience and have access to more data. This is highly useful in more complex domains such as healthcare and genomics.
The initial stages of the ML learning cycle often include three fundamental steps:
- The Decision-Making Phase
At its core, every ML algorithm is designed to analyze input data and generate an output, often in the form of a prediction or classification. Whether it’s labeled (supervised learning) or unlabeled (unsupervised learning), the system processes this data to uncover patterns or trends, such as identifying gene mutations that could lead to a rare disorder.
- Error Through a Loss Function Assessment
Once the model predicts, the next step is to get the prediction accuracy. This is achieved by employing a loss (or error) function. In cases where you know the correct result, the algorithm just compares its predictions to what happened, and that allows you to test how good it is and how far off it tends to be.
- Optimization and Learning Loop
The algorithm goes through an optimization cycle to improve its accuracy. It tweaks internal settings (or weights) so that it can measure the error and try again. This recursive process happens automatically, refining itself as it goes, until the model has reached the desired level of accuracy.
Importance Of Machine Learning In Rare Genetic Disorder Diagnosis
Rare diseases frequently affect small subsets of the population and present with overlapping or nonspecific symptoms. This is purely challenging to identify through routine clinical approaches.
But the digital revolution in healthcare, from better electronic health record documentation to genomic sequencing to wearable health tech, has made it possible for ML to:
- Process and interpret enormous, complex datasets in real time
- Discover latent patterns and associations between genes and disease
- Guess the diagnostic results when you know only some details.
- Speed up detection even with very few samples
Such capabilities are especially relevant in rare disease diagnosis, where lack of data, clinical variation. Also, fragmented health records have historically stood in the way of patients receiving treatment. We have listed a few ways in which Machine Learning has proven to be vital in rare genetic disorders:
- Genomic Sequencing and Variant Analysis
AI-based algorithms really can help to interpret whole-genome or whole-exome sequence data, especially to find rare pathogenic mutations.
- Challenge: Existing methods heavily depend on manual review by geneticists and sometimes produce “variants of uncertain significance.”
- ML Impact: There are tools (such as DeepVariant, SpliceAI, Exomiser) that use deep learning to be able to classify mutations and predict their functional impact with reduced time.
- Outcome: ML-based genomic diagnostics have markedly enhanced the diagnostic rate of rare diseases, and the technology even picks up causative variants missed by human reviewers.
- AI-Powered Diagnostic Imaging
The role of diagnostic imaging in the future of structural abnormalities, such as those found in genetic disorders (eg, skeletal dysplasias or structural abnormalities related to neurobiology), becomes clearer.
- Conventional Gap: Missed abnormalities can occur due to human error, interpretation delays, or information overload.
- ML Advantage: AI models such as Google’s DeepMind, Aidoc, and Qure. AI is sensitive to fine patterns in MRIs, CT scans, or PET scans that elude human eyes.
- Use Case: ML / AI-powered tools correctly identified >90% of people who had pneumonia or showed neurodegenerative markers, establishing their promise in rare disease screening.
- EHR-Based Predictive Analytics
ML models are able to glean useful diagnostic information from incomplete and fragmentary EHRs.
- What It Does: Scans patient history, prescriptions, lab reports, and symptoms to identify potential rare conditions via pattern recognition.
- Example: Tools like Phenotips and Face2Gene, and Phevor are using EHR phenotypes plus AI algorithms to pair patients with known genes or to flag cases for further investigation.
- Result: Time to diagnosis falls, as do tests that don’t need to be ordered.
- Clinical decision support systems (CDSS)
Intelligent CDSS systems use ML to facilitate the physician at the time of clinical examination by providing diagnostic recommendations, given a set of symptoms as input.
- Example: IBM Watson Genomics, BayesMendel, and FDNA use AI to analyze clusters of symptoms, match patient phenotypes to genetics databases, and recommend testing that’s relevant.
- Benefit: Assists GPs and non-specialists in detecting rare diseases sooner, reducing reliance on trial-and-error referrals.
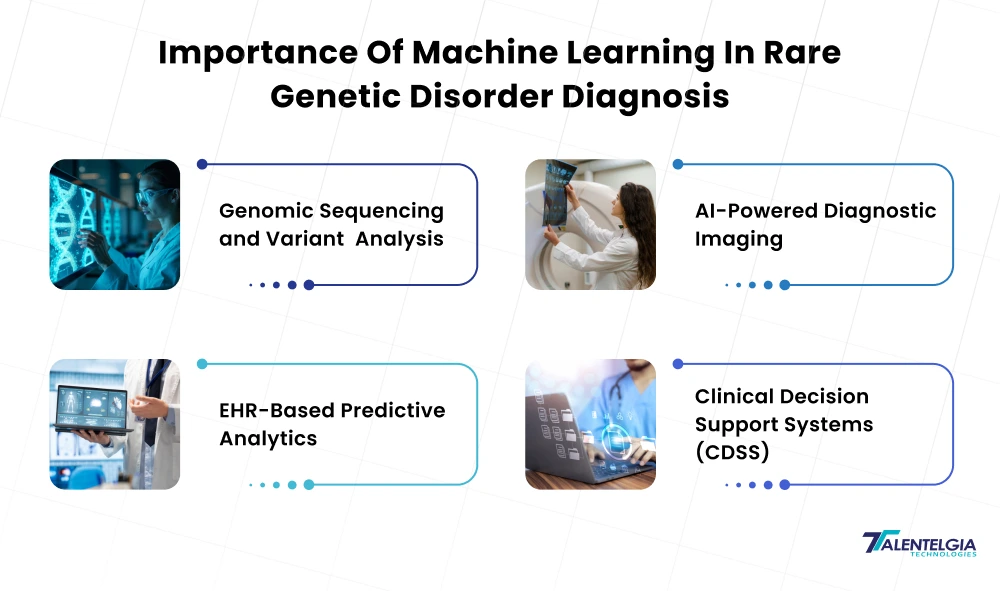
Challenges Of Using Machine Learning In Rare Genetic Disorders
Despite its transformative potential, machine learning is not without its hurdles, especially when applied to the diagnosis of rare genetic diseases. From limited data and privacy concerns to ethical implications, here are the key challenges that researchers, clinicians, and developers must navigate:
- Small, Biased Databases: The majority of rare diseases have only a very small number of recorded cases. There may be limitations and biases in the training data (e.g., some types of ancestries and phenotypes are oversampled). Models can become overfit or even fail on underrepresented groups. You will need to use your best tricks – data augmentation, transfer learning, or domain-specific knowledge. Stanford’s POPDx is an example of this: it relied on disease taxonomies and “few-shot” methods for predicting diseases not observed at training. However, model generalization will inevitably be tested by new cases or populations.
- Data Labeling and Quality: High-quality labels (the true status of a patient for receiving a rare disease) are difficult to find. They have mistakes or partial records, and that generates noisy labels. Chart review is resource-dependent. Most public datasets won’t have the depth (rich phenotypes, follow-up) for ML. Addressing these challenges requires semi-supervised approaches, such as active learning or international data exchange efforts.
- Privacy and Regulation: Genetic and Clinical Information is Very Sensitive. Regulations such as HIPAA (US) and GDPR (EU) heavily restrict how personal genomic information can be shared. To use patient data, they have to negotiate de-identification, informed consent, and data-use agreements. These restrictions often lead data to remain within the confines of a single hospital or country. New methods, such as federated learning (training a shared model without sharing any of your raw data,) are being actively researched to counter this.
- Explainability and Trust: Clinicians must comprehend ML outputs prior to action. Deep models are frequently “black boxes,” and tools for explainable AI (XAI) are essential. Techniques like SHAP or attention visualization can show what features (genes, labs, symptoms) were most important for a given prediction. Regulators and medical ethicists are also concerned with fairness: we can’t let AI be biased (e.g., systematically under-diagnosing minority populations). In health care, transparency about algorithm boundaries is as critical as accuracy.
Real World Applications Of ML In Rare Genetic Disorder Diagnosis
While machine learning holds great promise for the future, it is already transforming the way rare genetic disorders are detected and diagnosed today. Leading tech companies, research institutions, and healthcare innovators have developed powerful ML tools that support clinicians in identifying and treating complex genetic conditions with greater speed and accuracy. Here are some standout real-world examples:
- FDNA’s DeepGestalt (medical imaging AI): DeepGestalt uses 2D facial photos to prioritize genetic testing through identifying syndromic dysmorphology. Trained on more than 17,000 images of 200-plus syndromes, its deep CNN “quantifies similarities” between the facial characteristics of a patient and known phenomena. In a test, DeepGestalt had 91 percent top-10 accuracy in identifying the correct syndrome, from 502 such conditions. Clinicians use this application to help target genetic testing for patients with unusual faces.
- Google Health’s DeepVariant (variant calling AI): DeepVariant is an open-source deep learning comparative genomics algorithm that identifies the genetic differences between two genomes. As Google says, it “allows researchers and clinicians to compare an individual’s genome sequence-comprising the full 6.4 billion letters of an individual’s genome, with other genomes in Google Cloud Storage, to highlight genetic variations that may cause disease”. DeepVariant has achieved improvements in accuracy and consistency over classic callers by training on large benchmark datasets. Its family-aware extension (DeepTrio) extends de novo mutation detection power by utilizing both parents and the child. These are now used in research and clinical labs around the world to ensure better genetic diagnoses.
- Stanford’s POPDx and AI Ontologies: POPDx is a type of predictive model developed by researchers at Stanford, which uses a variety of data modalities and disease ontologies to predict diagnosis in the UK Biobank. In contrast to a lot of ML models, POPDx can detect diseases even outside the training chamber. Leveraging hierarchical disease information (e.g., Human Disease Ontology) and multi-label learning, the model improved its precision for previously unseen rare diseases by more than 150%. That would allow clinicians to screen vastly fewer patients to pick up those with a rare disease, doubling the chances of finding a low-prevalence case, say. These systems demonstrate that integration of clinical data, prior knowledge, and machine learning can open the door to predictive analytics in health care even for rare diseases.
Future Trends Of Machine Learning In Rare Genetic Disorder Diagnosis
As machine learning continues to evolve, its applications in rare genetic disorder diagnosis are set to become even more advanced, precise, and accessible. From integrating real-time patient data to enabling gene editing, here are the top emerging trends shaping the future of ML in rare disease detection:
- Personalized Medicine: ML algorithms can combine a patient’s genetic makeup with other data to suggest a therapy. For example, an AI might be able to predict how a patient will respond to a drug or provide recommendations for the best therapy to treat a rare metabolic disease. And as gene therapies (or personalized drugs, such as RNA therapies) progress, ML will continue to serve as a means of fitting the right therapy with each genotype. Stanford’s AI gene discovery work even has sights set on revealing new drug targets by mining literature and data. stanford. edumed. stanford. edu.
- Federated and Collaborative Learning: Getting around data silos with federated learning and privacy-maintaining ML will be a common phenomenon. Coalitions of hospitals or labs could train collective models without transferring patient data, increasing the effective size of the dataset for rare cases. This will speed progress without compromising patient privacy (one of the major trends in the future of AI in Healthcare ).
- Integration into Gene Editing: AI is already supplementing gene-editing technologies. For instance, ML models estimate CRISPR target sites or off-target risks. In the future, a rare variant found by an ML pipeline might be matched directly with a custom CRISPR therapeutic created by AI. This combination could potentially enable not just a prediction of genetic mistakes, but a correction, too. ML can further analyze high-throughput CRISPR screens (e.g., pooled guides in cell models) to identify novel disease genes.
- Continuous Learning and Real-Time Analytics: With increasing amounts of genomic-EHR data becoming available, learning can be continuous for ML models. Real-time predictive analytics in the clinic — for instance, warning a doctor that a patient’s symptoms so far on this and previous visits match a rare-disease signature — could become commonplace. The use of wearable and multimodal sensors (imaging, metabolomics) combined with genomic technologies will further enhance AI-based detection of rare diseases.
Quick Read: AI Use Cases In Healthcare
Conclusion
Advancements in machine learning promise to transform the chances of early diagnosis of rare genetic disorders. But if we unleash AI on genomics — in the form of deep neural networks that parse genomes, health records-mining algorithms that identify obscure but significant patterns — we can eliminate the long wait that patients must endure now. ML is no silver bullet: models have to navigate data inadequacy, pass the fairness test, and remain within privacy laws. But these early successes (more accurate diagnoses, faster discoveries, published tools) indicate real impact.
Rare diseases are now a global priority for the World Health Organization, calling for innovation and justice.
Unlocking ML’s promise will need clinicians, geneticists, data scientists, and regulators to work closely together. Responsible AI – in the form of explainable models, rigorous evaluation, and cross-disciplinary collaboration – will be crucial. Over the years to come, we’re going to see predictive analytics in healthcare fundamentally transform the diagnosis of rare diseases, identifying every piece of data that could be a clue that saves patients time and money from unnecessary suffering.


 Healthcare App Development Services
Healthcare App Development Services
 Real Estate Web Development Services
Real Estate Web Development Services
 E-Commerce App Development Services
E-Commerce App Development Services E-Commerce Web Development Services
E-Commerce Web Development Services Blockchain E-commerce Development Company
Blockchain E-commerce Development Company
 Fintech App Development Services
Fintech App Development Services Fintech Web Development
Fintech Web Development Blockchain Fintech Development Company
Blockchain Fintech Development Company
 E-Learning App Development Services
E-Learning App Development Services
 Restaurant App Development Company
Restaurant App Development Company
 Mobile Game Development Company
Mobile Game Development Company
 Travel App Development Company
Travel App Development Company
 Automotive Web Design
Automotive Web Design
 AI Traffic Management System
AI Traffic Management System
 AI Inventory Management Software
AI Inventory Management Software
 AI Software Development
AI Software Development  AI Development Company
AI Development Company  AI App Development Services
AI App Development Services  ChatGPT integration services
ChatGPT integration services  AI Integration Services
AI Integration Services  Generative AI Development Services
Generative AI Development Services  Natural Language Processing Company
Natural Language Processing Company Machine Learning Development
Machine Learning Development  Machine learning consulting services
Machine learning consulting services  Blockchain Development
Blockchain Development  Blockchain Software Development
Blockchain Software Development  Smart Contract Development Company
Smart Contract Development Company  NFT Marketplace Development Services
NFT Marketplace Development Services  Asset Tokenization Company
Asset Tokenization Company DeFi Wallet Development Company
DeFi Wallet Development Company Mobile App Development
Mobile App Development  IOS App Development
IOS App Development  Android App Development
Android App Development  Cross-Platform App Development
Cross-Platform App Development  Augmented Reality (AR) App Development
Augmented Reality (AR) App Development  Virtual Reality (VR) App Development
Virtual Reality (VR) App Development  Web App Development
Web App Development  SaaS App Development
SaaS App Development Flutter
Flutter  React Native
React Native  Swift (IOS)
Swift (IOS)  Kotlin (Android)
Kotlin (Android)  Mean Stack Development
Mean Stack Development  AngularJS Development
AngularJS Development  MongoDB Development
MongoDB Development  Nodejs Development
Nodejs Development  Database Development
Database Development Ruby on Rails Development
Ruby on Rails Development Expressjs Development
Expressjs Development  Full Stack Development
Full Stack Development  Web Development Services
Web Development Services  Laravel Development
Laravel Development  LAMP Development
LAMP Development  Custom PHP Development
Custom PHP Development  .Net Development
.Net Development  User Experience Design Services
User Experience Design Services  User Interface Design Services
User Interface Design Services  Automated Testing
Automated Testing  Manual Testing
Manual Testing  Digital Marketing Services
Digital Marketing Services 
 Ride-Sharing And Taxi Services
Ride-Sharing And Taxi Services Food Delivery Services
Food Delivery Services Grocery Delivery Services
Grocery Delivery Services Transportation And Logistics
Transportation And Logistics Car Wash App
Car Wash App Home Services App
Home Services App ERP Development Services
ERP Development Services CMS Development Services
CMS Development Services LMS Development
LMS Development CRM Development
CRM Development DevOps Development Services
DevOps Development Services AI Business Solutions
AI Business Solutions AI Cloud Solutions
AI Cloud Solutions AI Chatbot Development
AI Chatbot Development API Development
API Development Blockchain Product Development
Blockchain Product Development Cryptocurrency Wallet Development
Cryptocurrency Wallet Development About Talentelgia
About Talentelgia  Our Team
Our Team  Our Culture
Our Culture 
 Healthcare App Development Services
Healthcare App Development Services Real Estate Web Development Services
Real Estate Web Development Services E-Commerce App Development Services
E-Commerce App Development Services E-Commerce Web Development Services
E-Commerce Web Development Services Blockchain E-commerce
Development Company
Blockchain E-commerce
Development Company Fintech App Development Services
Fintech App Development Services Finance Web Development
Finance Web Development Blockchain Fintech
Development Company
Blockchain Fintech
Development Company E-Learning App Development Services
E-Learning App Development Services Restaurant App Development Company
Restaurant App Development Company Mobile Game Development Company
Mobile Game Development Company Travel App Development Company
Travel App Development Company Automotive Web Design
Automotive Web Design AI Traffic Management System
AI Traffic Management System AI Inventory Management Software
AI Inventory Management Software AI Software Development
AI Software Development AI Development Company
AI Development Company ChatGPT integration services
ChatGPT integration services AI Integration Services
AI Integration Services Machine Learning Development
Machine Learning Development Machine learning consulting services
Machine learning consulting services Blockchain Development
Blockchain Development Blockchain Software Development
Blockchain Software Development Smart contract development company
Smart contract development company NFT marketplace development services
NFT marketplace development services IOS App Development
IOS App Development Android App Development
Android App Development Cross-Platform App Development
Cross-Platform App Development Augmented Reality (AR) App
Development
Augmented Reality (AR) App
Development Virtual Reality (VR) App Development
Virtual Reality (VR) App Development Web App Development
Web App Development Flutter
Flutter React
Native
React
Native Swift
(IOS)
Swift
(IOS) Kotlin (Android)
Kotlin (Android) MEAN Stack Development
MEAN Stack Development AngularJS Development
AngularJS Development MongoDB Development
MongoDB Development Nodejs Development
Nodejs Development Database development services
Database development services Ruby on Rails Development services
Ruby on Rails Development services Expressjs Development
Expressjs Development Full Stack Development
Full Stack Development Web Development Services
Web Development Services Laravel Development
Laravel Development LAMP
Development
LAMP
Development Custom PHP Development
Custom PHP Development User Experience Design Services
User Experience Design Services User Interface Design Services
User Interface Design Services Automated Testing
Automated Testing Manual
Testing
Manual
Testing About Talentelgia
About Talentelgia Our Team
Our Team Our Culture
Our Culture
















 Write us on:
Write us on:  Business queries:
Business queries:  HR:
HR: